![[Translate to English:] Mischen und Homogenisierung von Produkten](/fileadmin/user_upload/micronisierung/micronisieren_mischen_und_homogenisierung_von_produkten.jpg)
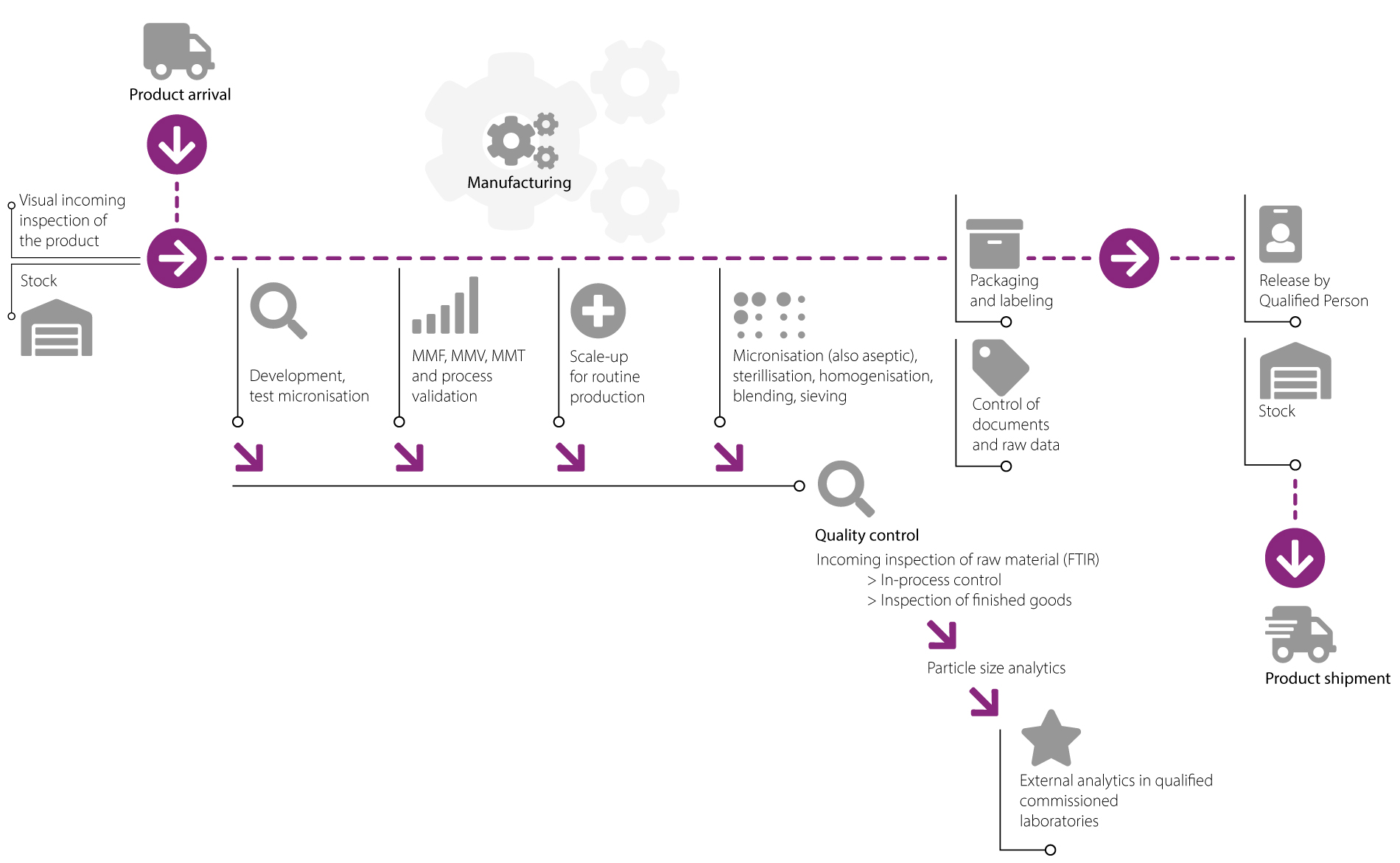
Micronization under cGMP conditions:
At GfM, we use our air jet mills (e.g. opposed and spiral jet mills) and mechanical mills (e.g. plate beater, pin and hammer mill) to perform micronizations in strict compliance with the cGMP guidelines (more than 30 mills available). From the first trial with just a few grams right up to tonnes, all with certified quality.
Aseptic micronization is an important process step in the manufacture of sterile pharmaceuticals which cannot be sterilized in their final container. This is one of our company’s competitive advantages.
![[Translate to English:] Prozessvalidierung](/fileadmin/user_upload/micronisierung/micronisieren_prozessvaidierung.jpg)
cGMP-conform micronization means having a valid process. We are pleased to offer this service by means of the validation of the product manufacturing process. Our service, in which all documentation for validation is closely coordinated with the customer and completed with a wealth of expertise, results in a quality standard which really pays off.
Whether for cleaning-, process- or measurement method validation, we are the right partner for your product.
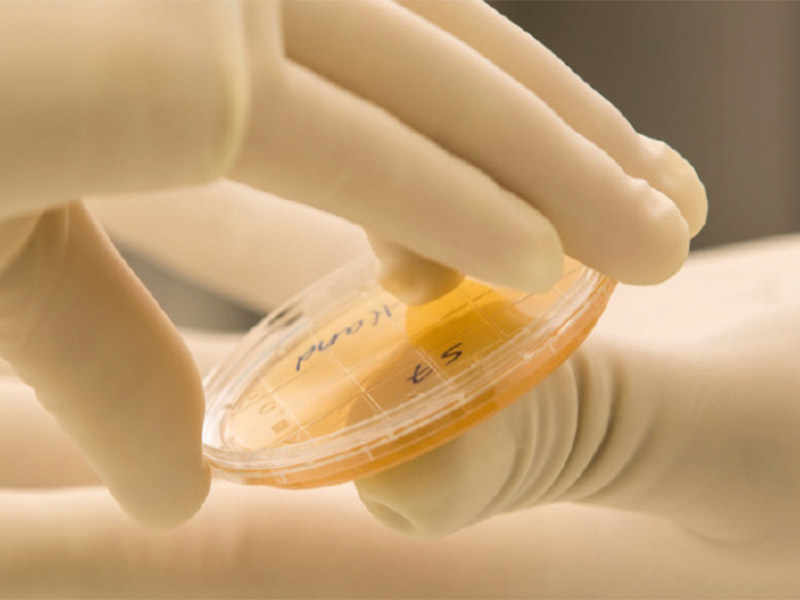
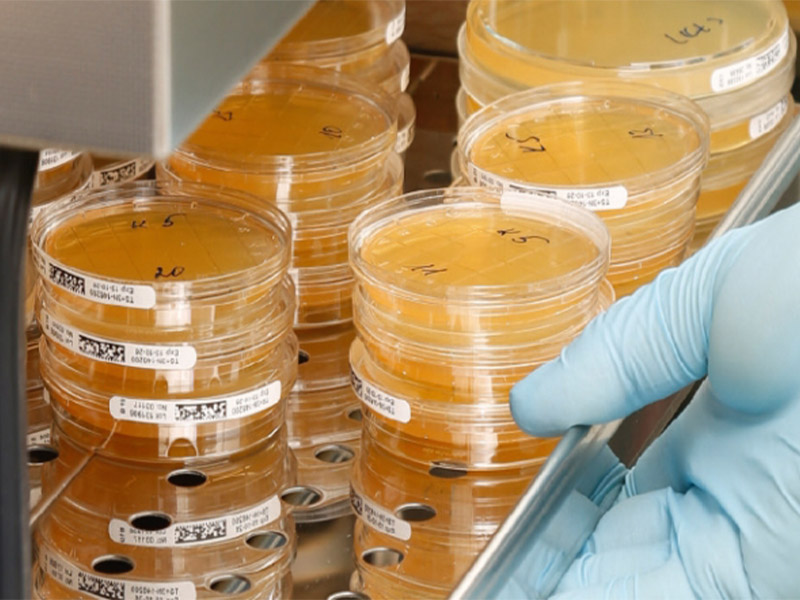
Microbiological monitoring of individual production areas includes surface contact tests (squeeze test), measurement of airborne germs as well as control of process air. Our production employees and production facilities are regularly checked by contact tests. In addition, measurements are carried out to determine the air particle count in the chambers and the process air. Trend analysis are performed to ensure long-term compliance with the standard.
Our success is based on the consistent implementation of cGMP guidelines regularly confirmed by the state authorities of Bremen, Germany.
The central key factor behind our business success is product quality. The main building blocks for this are the continuous implementation of hygiene plans as well as product group-specific storage and manufacturing (dedicated equipment) of the active ingredients and excipients.
As our service range includes the processing of potent pharmaceutical products, we guarantee a high level of safety for our employees. This is possible, among other things, thanks to the use of functional protective clothing.
Regular in-house training complemented with external advanced training ensure that our safety measures are always up-to-date.
![[Translate to English:] Produktbezogene Siebverfahren](/fileadmin/user_upload/micronisierung/micronisieren_produktbezogene_siebverfahren.jpg)
![[Translate to English:] Produktsterilisation](/fileadmin/user_upload/micronisierung/micronisieren_produktsterilization.jpg)